Calculate The Equilibrium Pressure Of N2o4 When The System Reaches A New Equilibrium
Calculate the equilibrium pressure of n2o4 when the system reaches a new equilibrium. The volume of the container is doubled at constant temperature. For a gas-phase reaction the expression for is. For the second equilibrium since the volume of the container is doubled the total pressure will be one half ie 2 1.
And our piano too is one point one atmospheres. And so now at our new equilibrium our pressure of end two o four is our zero point one four minus x so zero point one four minus zero point zero four five that equals zero point zero nine five atmospheres. An equilibrium was established at a constant temperature according to the following equation.
At equilibrium the partial pressure of O2 is. Calculate the equilibrium pressure of NO2 when the. The volume of the container is doubled at a.
An equilibrium mixture contains N2O4 P 027 and NO2 P 10 at 350 K. Calculate the equilibrium pressure of N2O4 and NO2 When the system reaches a new equilibrium. A Write the mathematical expression for the equilibrium constant.
The effect of pressure on equilibrium - N2O4 to 2NO2. Aaq 2Baq 2Caq Kc 1 103. 2 posts Page 1 of 1.
An equilibrium mixture contains N2O4 P 027atm and NO2 P 11 atm at 350 K. N2O4 when the system reaches a new equilibrium. 6 9 a t m The partial pressures of N 2 O 4 and N O 2 at equilibrium will be 0.
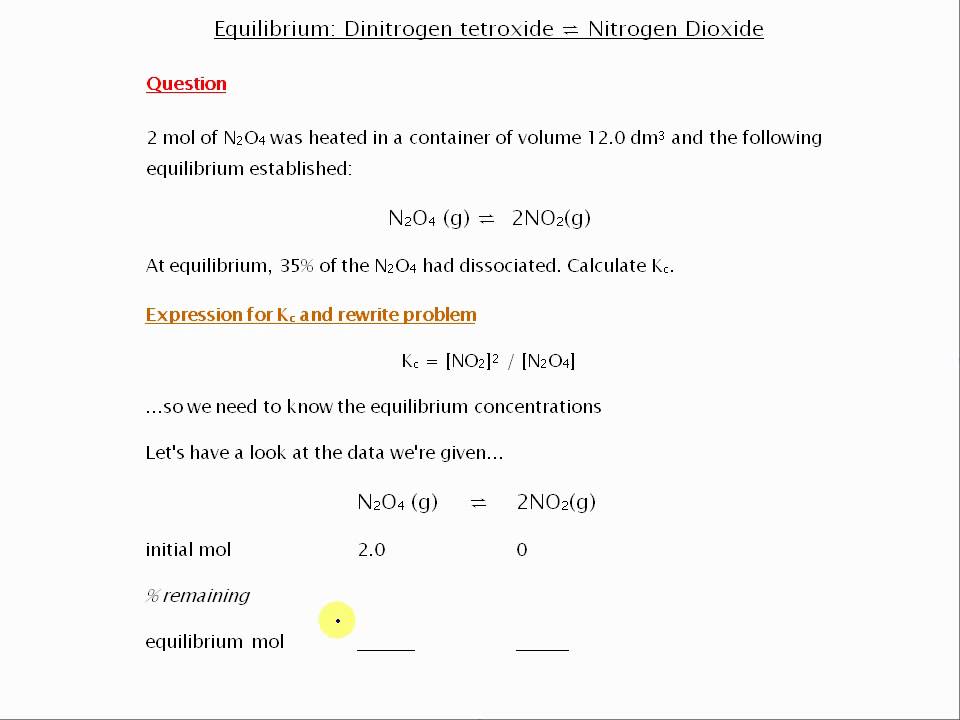
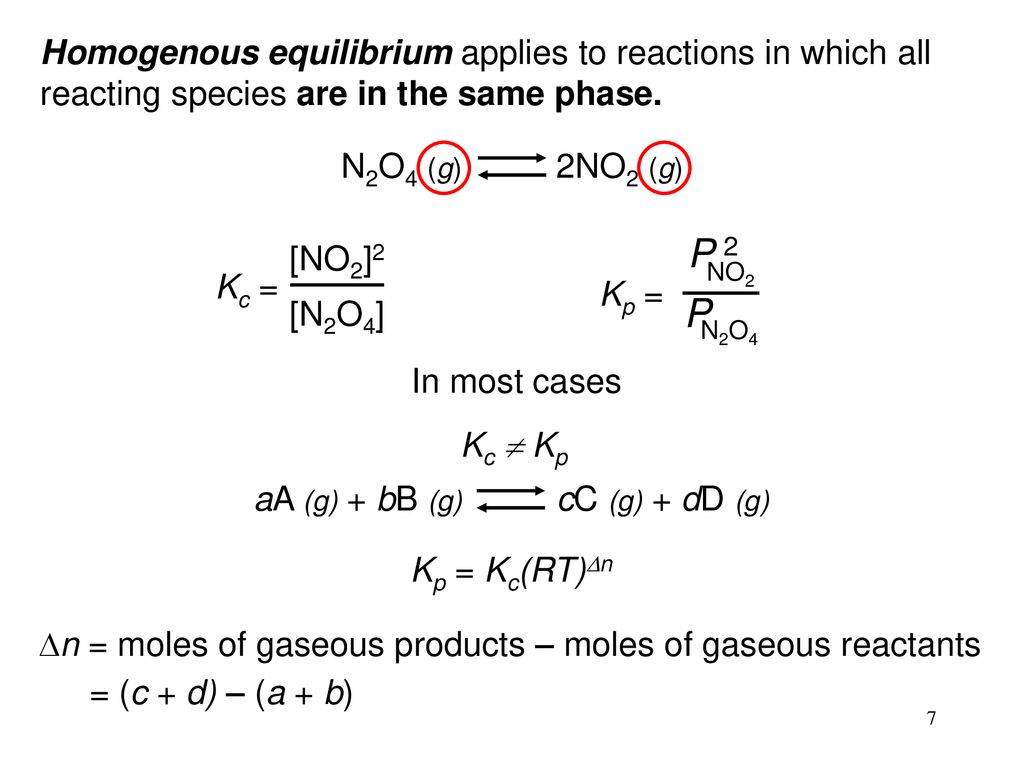
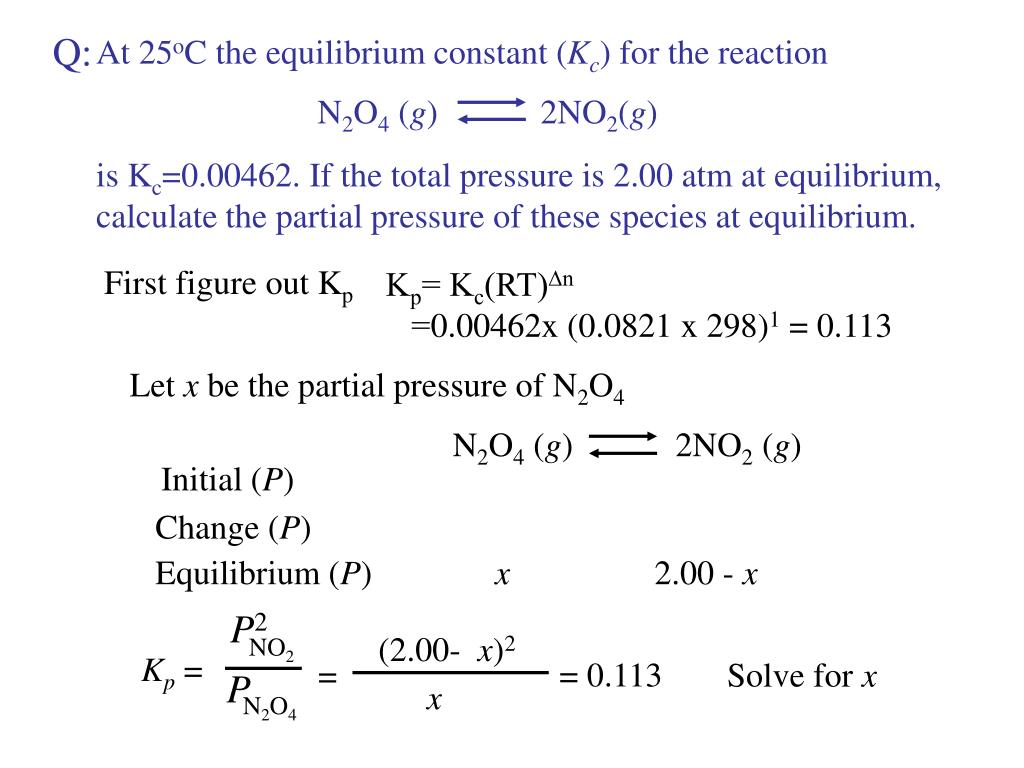
Is related to the equilibrium constant in terms of molar concentration by the equation below. 2NO2 gN2O4 g When the system is at equilibrium it contains NO2 at a pressure of 0714 atm and N2O4 at a pressure of 00510 atm.
K p p r o d u c t s r e a c t a n t s K p 314 10 16 123 10 4 2 208 10 8.
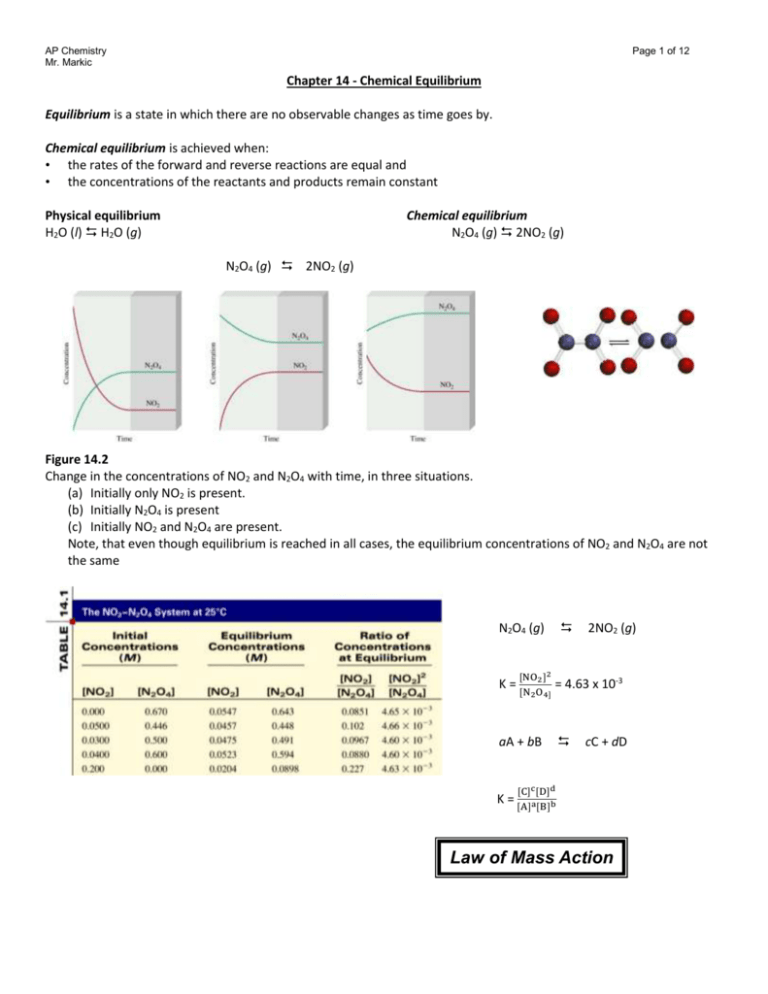
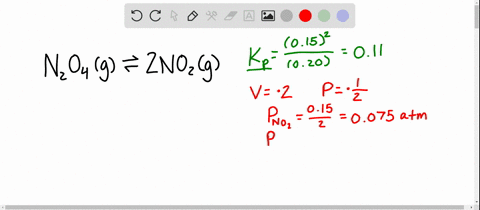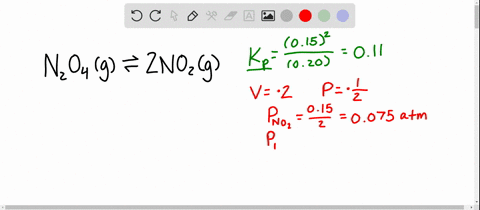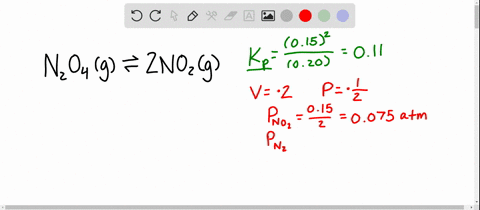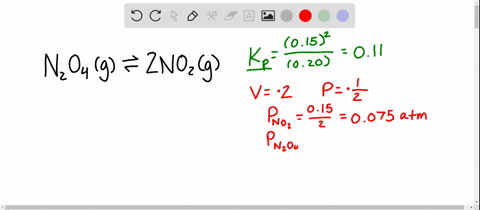
An equilibrium mixture contains N2O4 P 027atm and NO2 P 11 atm at 350 K. The equilibrium constant describes the ratio of product and reactant concentrations at equilibrium in terms of partial pressures. N2O4 when the system reaches a new equilibrium. A Calculate the equilibrium pressure of. The value of the equilibrium constant will be. Is related to the equilibrium constant in terms of molar concentration by the equation below. 6 9 a t m The partial pressures of N 2 O 4 and N O 2 at equilibrium will be 0. The equilibrium partial pressure of each reactant will be the given initial partial pressure minus x. And so we can calculate R K P which is just p N O two squared over p and two o four so one point one squared over zero point.
The volume of the container is doubled at constant temperature. An equilibrium mixture contains N2O4 P 027 and NO2 P 10 at 350 K. So in this equation we have the reaction and two oh four gas react Reverse Italy to form two and O two gas. The volume of the container is doubled at constant temperature. P H 2 O P t o t a l P H 2 0016 0013 a t m 0003 a t m. Calculate the equilibrium pressure of NO2 when the. P SO2 0150 x 0150 0013 0163 atm P NO2 0200 x 0200 0013 0213 atm.

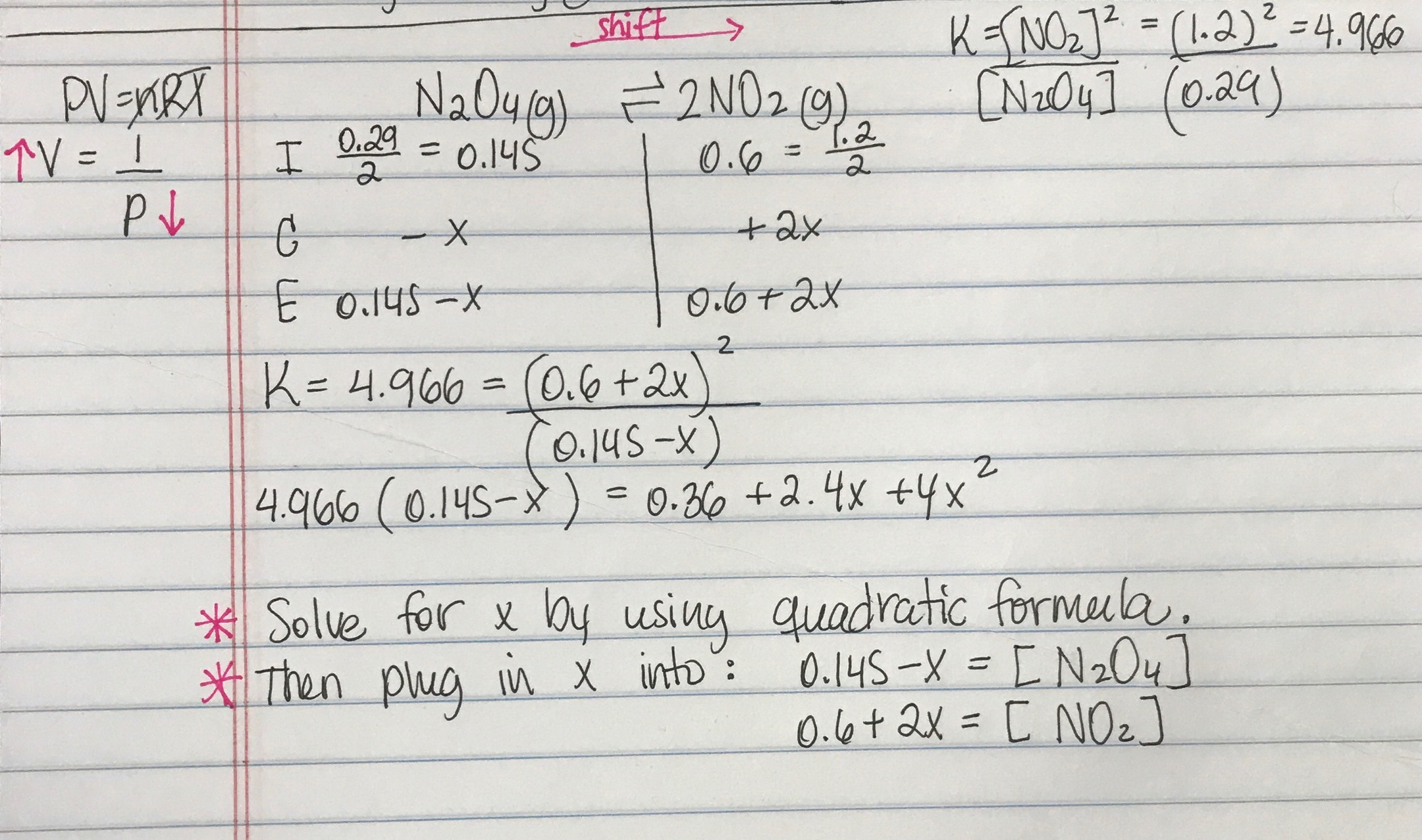















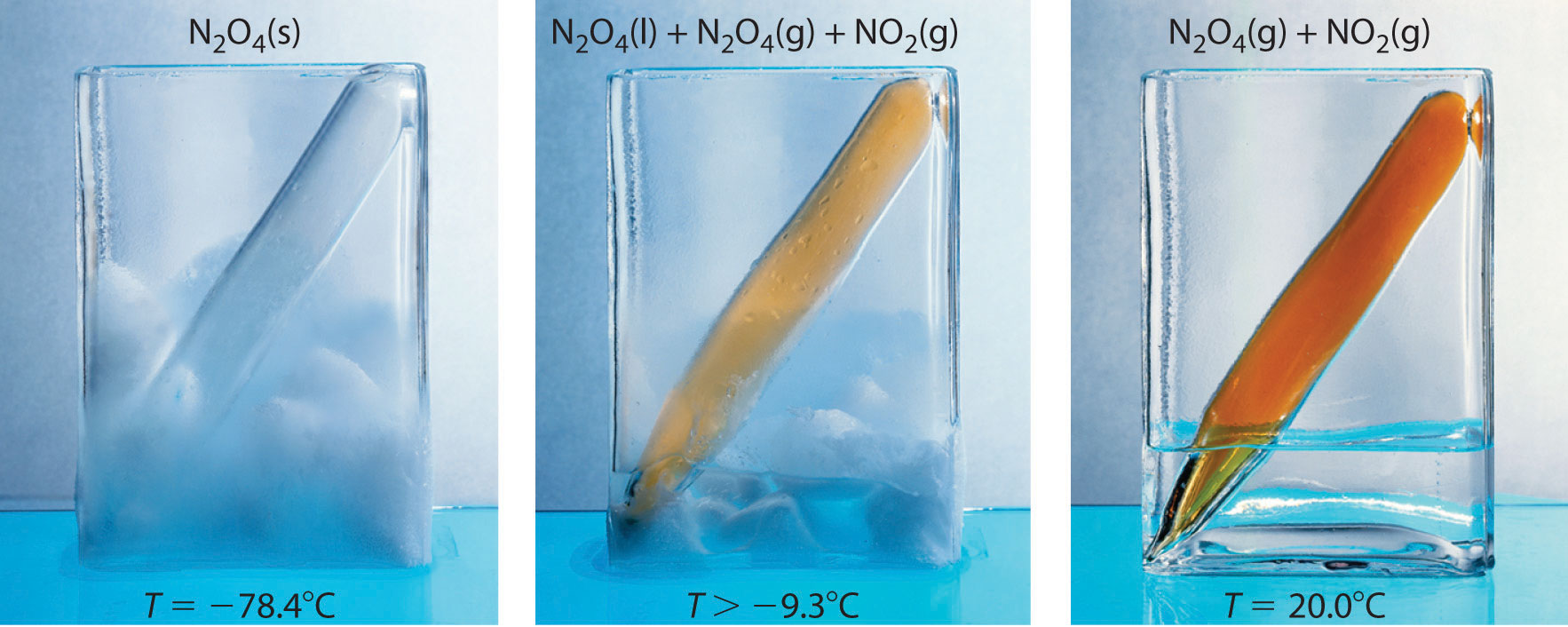




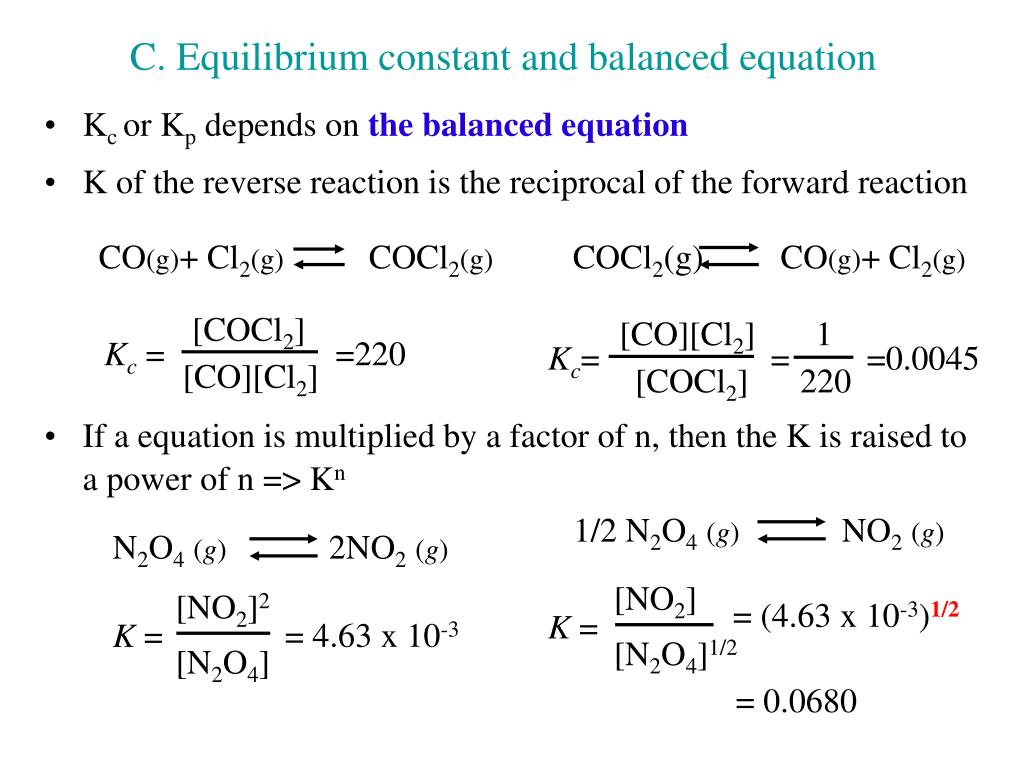





Post a Comment for "Calculate The Equilibrium Pressure Of N2o4 When The System Reaches A New Equilibrium"